
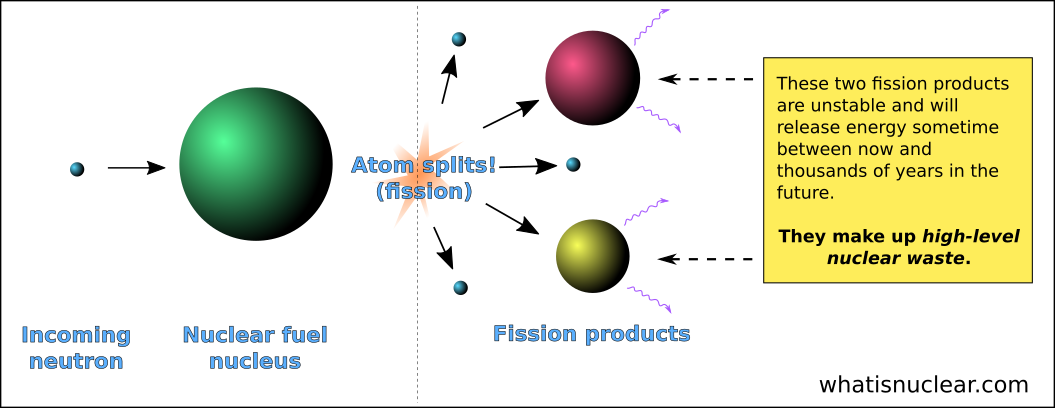

This example uses the fission of U-236, a nuclide not often used in fission examples. Personally, I don't care too much for this formatting style, but you do see it used on the Internet a fair amount. The atomic number calculation looks like this:Īnd the mass number calculation looks like this:įor these last two examples, I will use alternate formatting styles.Įxample #9: My preferred alternate formatting style:ĩ2-U-235 + 0-n-1 -> 57-La-148 + 35-Br-85 + 3(0-n-1)Įxample #10: A formatting style I have seen used:Ģ36/92 U + 1/0 n -> 100/42 Mo + 126/50 Sn + 11(1/0 n) When you total up the atomic numbers, use −1 for the electron's "atomic number" and 0 for the mass number. That β¯ stands for a beta particle, which is known to be an electron. There's also a 92 236 U example below (at #10).Įxample #6: Here's another 92 233 U example: The most common nuclide used for fission reaction examples is 92 235 U, but you do see the two just above from time to time. I put ones in there to remind you about the neutrons.Įxample #4: Here is another fission reaction: On the right-hand side of the first equation, we have this: In all three cases above, the total mass number on the left-hand side is 236. The mass numbers have to add up to be equal on each side of the arrow. I put zeros in there to remind you about the neutrons. In all three cases above, the total atomic number on the left-hand side is 92. The atomic numbers have to add up to be equal on each side of the arrow. Two Important Rules About Writing Fission Reactions Energy will be involved in some examples below, starting with #14.Įxample #3: Sometimes, the nuclide that is formed for a brief instant before fissioning, is shown: I plan to ignore the energy term in much of what I write below. MeV is a common measure of energy used in nuclear reactions.Įxample #2: Often, in the writing of a fission equation, the energy (usually in the form of gamma radiation) released is ignored. Sometimes the word energy is used and you will also see MeV (megaelectron volts) used. Sometimes, γ (Greek letter gamma) is used. Q stands for the nuclear energy produced. Examples that must be solved start at #11.Įxample #1: Here is a typical fission equation: The first ten examples are different fission reactions with some comments attached here and there. I do not want you to draw the conclusion that only the elements barium and krypton show up as fission products. I did that to demonstrate that more than one isotope of a given element can show up as a fission product. The first three fission equations all use isotopes of barium and isotopes of krypton for the products. You can read a bit more about the discovery of fission here and here. My goal here was only to give you a brief look at the relevant events. Obviously, work relevant to the discovery of fission took place before 1932 and fission continued to be studied after 1938. Meitner, who had work closely with Hahn on fission, had been forced to flee Nazi Germany in July 1938 and had found a job in Stockholm. This work was carried out by Lise Meitner and Otto Frisch. The explanation for the physical mechanism of fission took place over the Christmas holiday of 1938, in Stockholm. The discovery of nuclear fission took place in early December 1938, in Berlin, by Otto Hahn and Fritz Strassmann. About 1934, he thought he had discovered new elements beyond uranium, however he had fission take place, but did not recognize it as such. One of those places was in Rome, the work being headed up by Enrico Fermi. More or less immediately, the neutron was seen as a tool to probe the nucleus of the atom and work commenced in many places. The neutron was discovered in 1932 by James Chadwick. ChemTeam: Fission Nuclear Reactions: FissionĪ Brief Tutorial About Writing Nuclear Symbols


 0 kommentar(er)
0 kommentar(er)
